Originally shared on Sana’s LinkedIn Profile.
The FDA and EMA appear to be accelerating the QbD push, following the suggestion in 2012 that ANDAs for generic drugs should, in fact, have QbD elements in them.
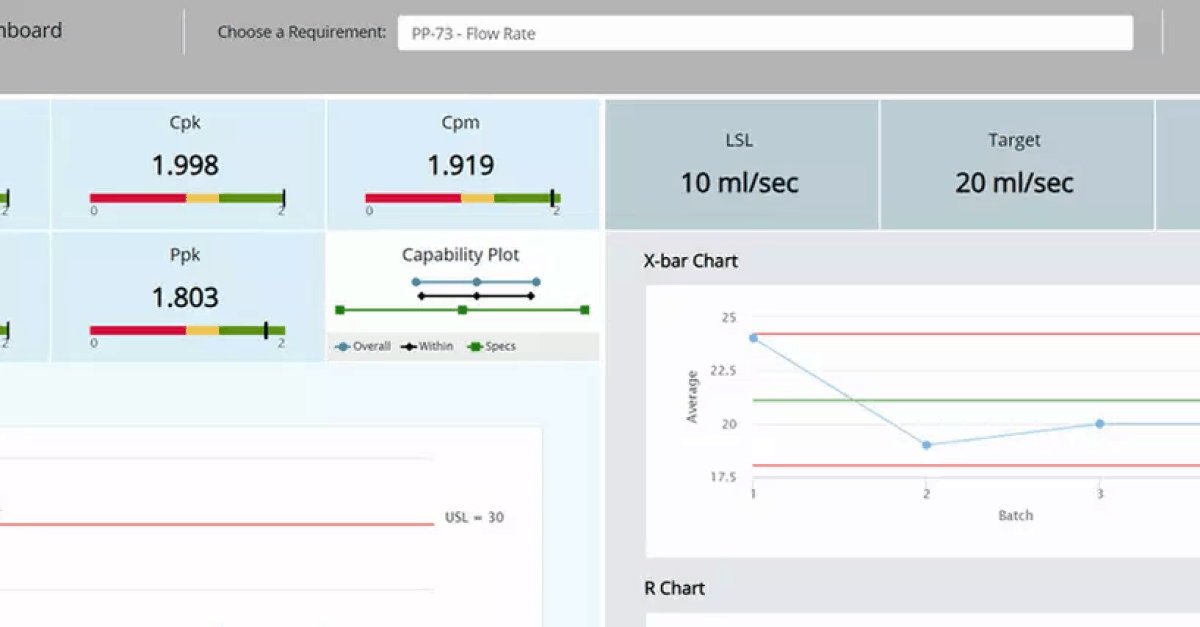
Quality by Design in itself, as a concept, is not new in my opinion – and it seems many other industry scientists agree. The process of building quality into your manufacturing method and product is not simple; however, what QbD does, is simplify the complexity that it involves. QbD provides a ‘streamlined approach.’ It is a series of steps, leading you to an outcome where you are omnipresent throughout the lifecycle – without physically having to be there, anxiously watching over your project.
But how does that work? By putting the work in first, rather than ‘fixing’ and ‘treating’ once the API hits the fan, so to speak. With the right toolset, team, and target – a structured and consecutive approach is made more accessible. The right tools should allow you to record, screen, store, manage, and analyze scientific data that builds up quite rapidly throughout the lifecycle. From concept to clinical, all the way through to commercialization. The right team should have clear and strategic communication channels that allow information acquired, during the manufacture and release of the product, to be relayed with appropriate turnaround time. Strategy with communication at the project planning stage is critical, as more often than not, you or your team members will juggle various projects at a single time.
The right target should always revolve around the patient. Having a patient-orientated target, allows you to aim for the formulation and production parameters, that you need to achieve said target – and this is justification for your cause. Ever wondered what the justification column was for on (Q)TPPs?
The right target should always revolve around the patient. Having a patient-orientated target, allows you to aim for the formulation and production parameters, that you need to achieve said target – and this is justification for your cause. Ever wondered what the justification column was for on (Q)TPPs?
Having a process related target does have value, and in the development stages, it is necessary – for both the formulation and process. But this will only take you so far if you’re not aware of the impact your manufacturing range is going to have on your TPP/QTPP and ultimately, the patient. In some cases, patient targets are stumbled upon, after in-depth Root Cause Analysis, due to a deviation from the method. This is a waste of time as a large number of practical investigations are done in method development, and the outcome from those stages should be robust enough to prevent associated parameter failures further down the production line.
Knowing your process or formulation is ‘outside the region’ is not enough. Making these links between your requirements, parameters, results, and data – is seen as time-consuming to some, but beginning a project without connecting the dots is going to result in those connections being made by default, through deviations and change controls. So, it just depends on whether you want to sit behind the wheel or navigate from the passenger seat…