In Season 5 of the QbDVision Software Release Series, we introduced you to an expansion of a number of our newest feature sets, such as QTPP Management, Multiple Process Definitions, and more streamlined Data Management.
With the release of Season 6, you can put on your dancing shoes as we continue to expand the feature sets to streamline your key workflows and advance the theme of Digital CMC within your organization.
Episode 1: Bringing Data Together With Integrity
Did you know that pharma/biotech companies only use about 4% of their data on average? Yes, it’s true, but probably not surprising to many of you with the prevalence of information silos in many organizations.
In our last release cycle, we featured the new changes to our Data Management module to simplify how your raw material and manufacturing data can be logged and tracked in QbDVision. Key new features include:
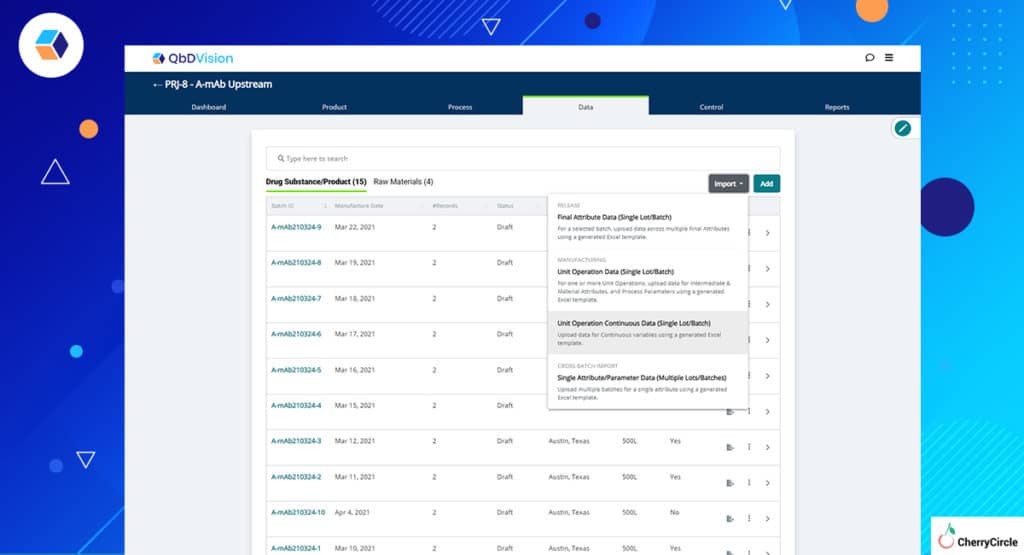
- New Import Workflow – Getting your batch and raw material data into QbDVision is now easier with new import workflows accessible right from the batch or raw material record created to hold this data.
- Template Generation – Need a template to get your data imported, access the template generation feature directly from the batch or raw material record as well.
- Visualization of Imported Data – We have further simplified visualization and tracking of these data by creating lists showing which data is from DS/DP batches and which is CoA data categorized by associated material.
- Data Integrity – We have always focused on ensuring that our data workflows conform to the best practices of data integrity by design. For any set of data imported into QbDVision, supporting documents such as original batch records can be attached along with the imported data templates to ensure full traceability back to the source data.
Episode 2: Using Your Data, Finally!
So, you have all of this great data coming together in one software platform. Now what? Data analytics is all the rage these days. But, we think that Information Analytics is a lot more powerful. What is Information Analytics? Well, Information = Data + Context and QbDVision brings these two together to facilitate information management. Maybe the reason that data utilization is so poor is because it lacks context. QbDVision allows you to go one step further and capture the evolution of the information over time so that it becomes institutional knowledge. Whoa!
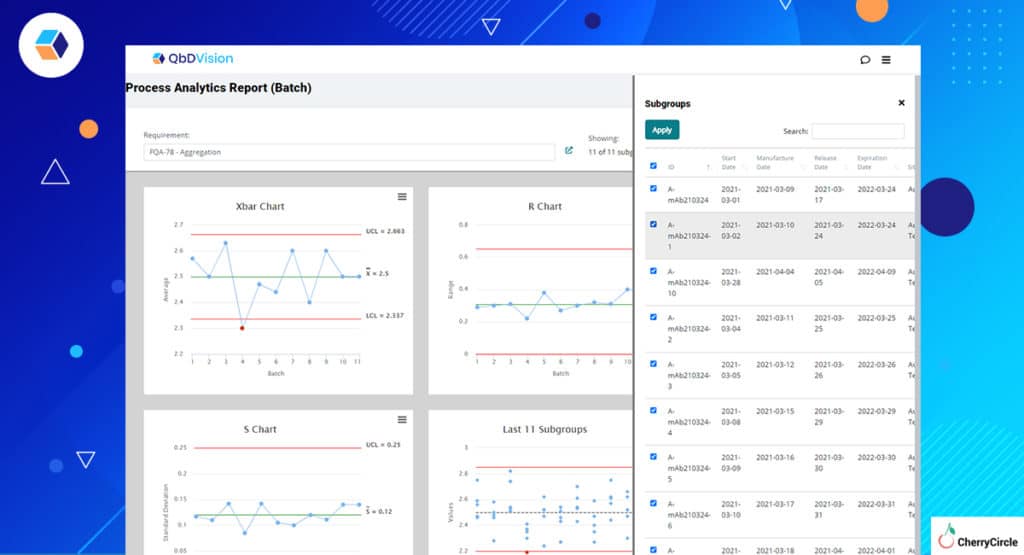
- Manufacturing Analytics – We have always brought context to data within the QbDVision platform and Flamenco has refined this even further. Now you can select specific batches of data on which to run your analyses. Want to see how one subset of batches is performing relative to another for a particular attribute or variable? Now you can do that easily in QbDVision and use that information to adjust the Occurrence risk for the attribute or parameter of interest.
- Raw Material Analytics – QbDVision gives you the ability to capture raw material attribute data directly from CoAs. As you log data from more and more vendor lots, data from each new lot can be compared against previous lots to flag you if something seems amiss.
Start using Information Analytics so you can evaluate process performance and make intelligent assessments about process risk and characterization within the same environment.
Episode 3: Prime Time for Process Management
One of QbDVision’s key value propositions has always been the management of the evolution of your manufacturing process over the development lifecycle. In this release, we have added two key feature sets that deepen this capability.
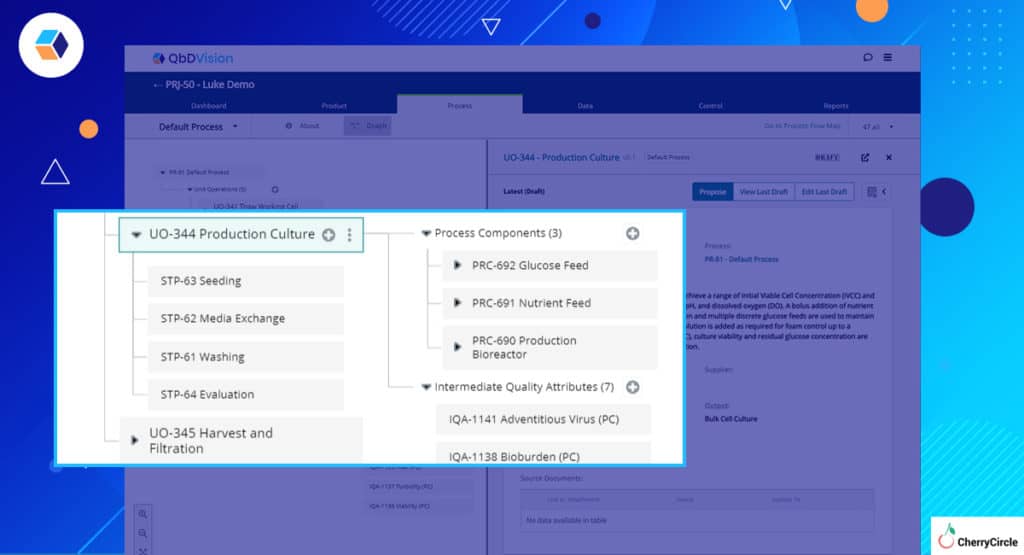
- Process Steps – Process management in QbDVision has centered around the definition of Unit Operations to categorize groups of activities. Our users have been asking for the ability to go more granular with the definition of Process Steps within each Unit Operation and now they can. Breakup each Unit Operation into as many Steps as you want.
- Global vs. Local – Now with the additional layer of Process Steps, materials and process components and their associated attributes, parameters, in-process controls, and quality attributes can be defined globally at the Unit Operation level or locally at the Step level.
- Keeping It Simple – Say you are baking a cake and you know that oven temperature is important but you don’t know what oven you are going to use? This happens all the time in pharma/biotech manufacturing. QbDVision allows you to define process parameters for Unit Operations and Process Steps without having to define the tangible asset of production associated with the process parameter.
- Multiple Process Imports – With the ability to define multiple manufacturing processes for each product came the need to import information into each process separately. QbDVision now supports the import of both structural process information and process data into the desired process.
This just keeps getting better!
Episode 4: Audience Favorites
We are big believers in making sure our customers are happy and there is no better way to do that than to show them we are listening. You can always make a feature request through our customer support portal and we wanted to highlight some of the changes to the QbDVision platform that were requested by our loyal users.
- Archiving Without Approval – We understand the critical importance of data integrity which is why the archiving of information is a key functionality. In the past, archiving a record required approval by another party in the organization. For records that are approved via electronic routing, this is still the case. But, we all make mistakes, and records still in a Draft state can now be archived without another users’ approval.
- Failure Modes for Material Attributes – When we designed QbDVision, we included a Failure Modes field for our Process Parameter data type. We knew this would be helpful when automatically generating FMEA reports in the future (yes, there is a better and more compliant option than manually creating and updating FMEA spreadsheets). Our users loved this feature so much, they wanted us to add the Failure Modes field to the Material Attribute data type as well. So we did! In the next release, we’ll add Material Attributes to the FMEA too!
- Retraining Request – QbDVision also integrates many elements of eQMS such as document management, training management and supplier and risk management. There are times when an employee needs to retrain on a document for which they previously completed training. In this release, we have added the capability to easily request and track the re-training of an employee on a controlled document.
The ramifications for simplifying tech transfer are HUGE! Define all the elements of your process at one site/scale (requirements, variables, materials, equipment, etc.) and then quickly copy and modify for another site/scale. With the click of a button, you will generate future reports comparing the differences and completing in-line risk assessments on these differences. Wow! The process “neighborhood” is starting to look pretty good!
Season Finale: Enabling Digital Tech Transfer
The features highlighted in the previous episodes have an impact beyond their specific functionalities. In other words, there is a method to our madness. All of the new features of Flamenco come together to enable a new workflow with significant benefits – digital tech transfer. Many of you in the industry are familiar with the challenges and frustrations of trying to execute tech transfer in compressed timeframes. The document-centric approach to transfer information from sending sites to receiving sites is still heavily used but obviously antiquated in today’s digital world. The structured data framework which is the foundation of the QbDVision platform is a fit-for-purpose architecture for digital tech transfer. QbDVision is now the first software solution built as a fit-for-purpose solution specifically for the tech transfer needs of the pharma/biotech industry.
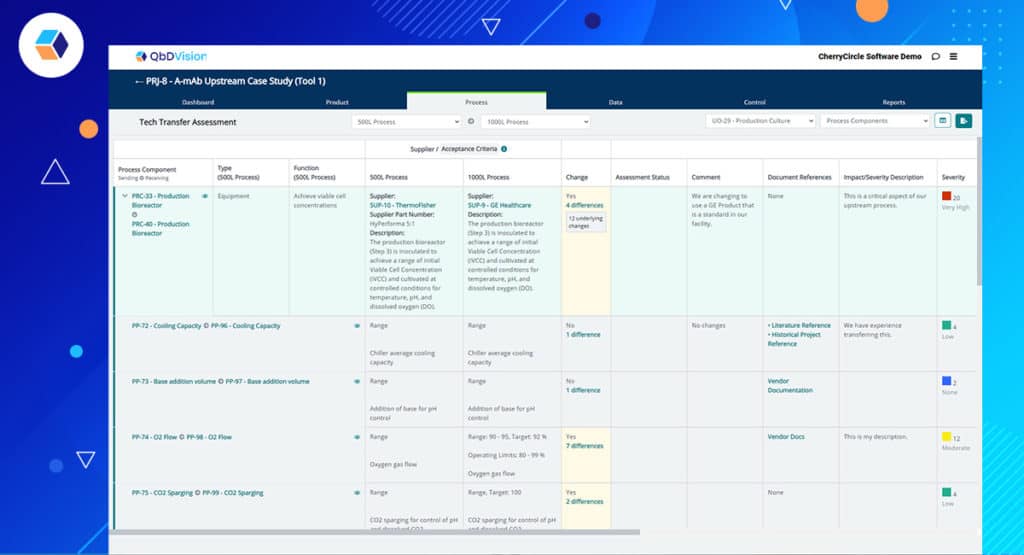
Key features of this solution include:
- Digital Initiation – Initiate the tech transfer process by instantly copying the sending site process into receiving site process. Now you have two processes where each element of the sending and receiving site processes are linked together.
- Process Editing – Modify the receiving site process to record different equipment, process parameters, acceptance criteria, etc.
- Real-Time Gap Assessment – With two highly structured and linked processes managed within the same environment, automatically generate interactive gap assessment reports. Since your processes may continue to change and evolve, the gap assessment can evolve in real-time as well.
- Real-Time Risk Assessment – With the gaps identified between the sending and receiving site, perform your risk assessments for each gap within the same workflow in QbDVision and record the next steps to further characterize the gaps and mitigate the risks.
- Track Supporting Documentation – The ability to attach links and upload documents to any record type is a key feature of QbDVision and this feature displays prominently in the digital tech transfer workflow where large volumes of information are being managed and rapid access to supporting documentation is critical.
This is just the beginning of the digital tech transfer revolution. The power of this approach will become even more evident as we build more automation into these workflows. Stay tuned!
If you are new to QbDVision and interested in trying out our solution, it’s available for a free 30-day trial, or reach out to learn more about exploring the platform’s expanded capabilities.