EDUCATION
Build your expertise with us
A curated library for your CMC teams
Best practices
Hear experts provide their insights on drug development topics.


WEBINAR
Improving Final Product Quality by Embracing Knowledge Management
See why a risk-based approach is best to allow development within a reasonable time and budget while maximizing safety and efficacy.


WEBINAR
ICH and Pharma 4.0: Implications for Brazil
Learn how recently adopted ICH guidelines impact key pharmaceutical manufacturing stakeholders in this growing market.


WEBINAR
Accelerating QbD: Leveraging ICH and Digitization for Success
Industry experts share their perspectives on how to use the quality target product profile (QTPP) as the foundation for designing quality into a product.


WEBINAR
2019 QbD Symposium with Yash Sabharwal
Watch our CEO’s featured presentation, “Risk-Based Knowledge Management, Spatial Analytics, and Data Integrity.”


WEBINAR
Austin A-List Winner: CherryCircle Software
See why our parent company was recognized as one of Austin’s most innovative homegrown companies by the Austin Chamber of Commerce.


WEBINAR
Intro to ICH and QbD Principles for biopharma
Sana Ahmed, QbD and Client Services Specialist at CherryCircle Software, discusses the intersection of ICH and quality by design (QbD) principles.
Articles
See what best practices drug development experts are discussing.
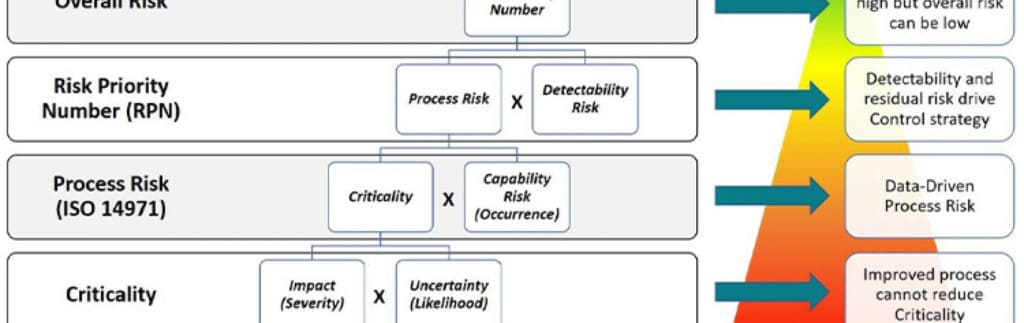
See why industry leaders think it’s time we laid these outdated tools to rest.
How to define them in a biopharma knowledge management setting.

How to prepare your manufacturing processes for success in the next big stage.
Whitepapers
Strengthen your expertise in knowledge and quality risk management.

See how raw material variability can affect your process and final product quality.

Get a practical perspective on how to rationalize and standardize risk assessments.
Books
Dive deeper into key drug development topics.

Explore practical approaches to building quality into your processes.

Get in-depth insights into Into pharmaceutical processes, management, and regulatory affairs.

Learn about the latest best practices in controlling the output of drug development processes.
Podcasts
Listen to thought provoking discussions about drug development.

Explore an archive of insights from some of the rockstars of life sciences R&D.
Key authorities
Get quick access to the latest international guidelines and regulations.

US Food and Drug Administration (FDA)
Relevant Links

International Conference on Harmonisation (ICH)
Relevant Links

Pharmaceutical Inspection Co-Operation Scheme (PIC/S)
Relevant Links
Harness your expertise
See how QbDVision can help you leverage your team’s know-how to accelerate drug development.
























































