What’s Changing
We’ve added a new record type called a General Attribute (GAs) as part of the definition of the Quality Target Product Profile (QTPP).
The QTPP forms the basis of design for the development of the product. The GA record type has a structure like a Target Product Profile (TPP) element and is designed to allow for the definition of general product attributes such, intended use in clinical settings, route of administration, dosage form, delivery systems, dosage strength(s), etc. (ICH Q8 Annex Sec. 2.1).
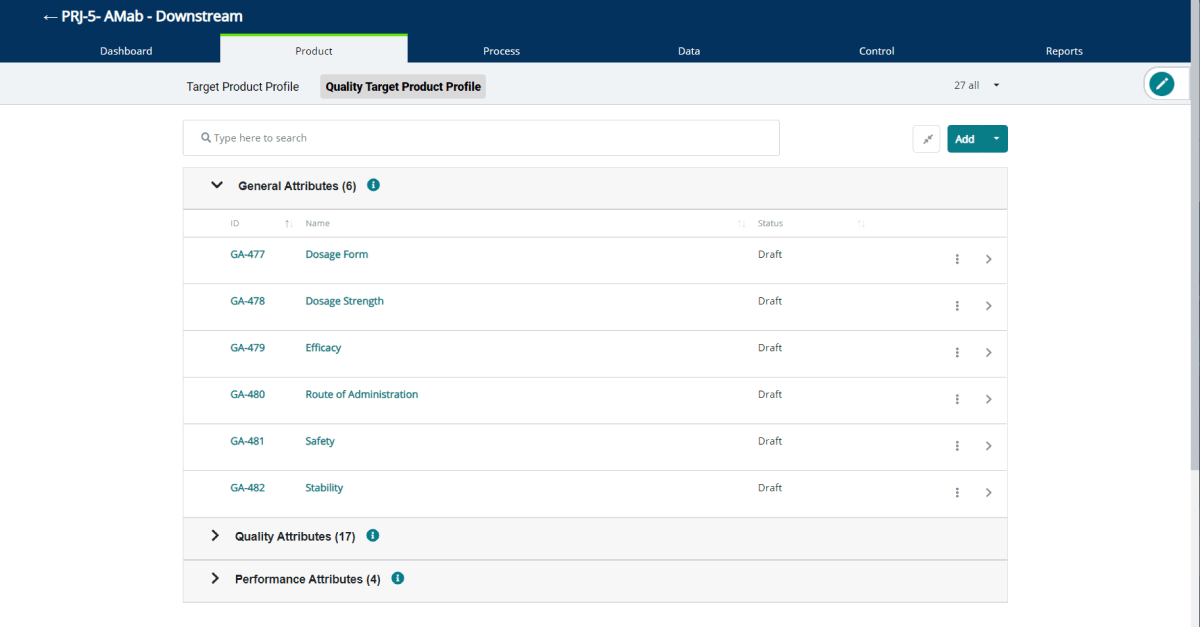
QbDVision automatically creates the following GAs for you when you create a new project:
- Dosage Form
- Dosage Strength
- Efficacy
- Route of Administration
- Safety
- Stability
Existing projects will now see a new section that is blank for General attributes in the new Product section.
The addition of the GA record type unlocks significant new functionality with respect to risk assessment (ICH Q9). For biologics, it is often desired to risk rank product quality attributes against Safety and Efficacy. With this expanded functionality, QbDVision allows for the definition of Safety and Efficacy as GAs and the risk assessment of quality attributes against these GAs. For example, you can specify that Sterility (a Quality Attribute) has a high impact on Safety, but a medium impact on Efficacy providing more granularity with your risk assessments.
If your product risk assessment process doesn’t fit into such a granular risk ranking system, then you can still use our original preliminary hazard analysis (PHA) method and simply link your quality attributes directly to GAs (without assigning a risk level).
Why is this happening?
Our initial QTPP functionality focused on quality attributes as the primary level of concern. However, as a definition of product requirements intended to form the basis of design for the development of the product, the QTPP should include attributes relevant to patient safety and efficacy along with the ability to complete risk assessment of the quality attributes against safety, efficacy, and other relevant attributes.
This new functionality significantly expands the existing capability of QbDVision making the QTPP definition more comprehensive.
How do I use it?
In the realm of biologics drug substance and drug product development, it is important to assess the risk of quality attributes against the more detailed patient and safety efficacy attributes.
As an example, the A-mAb case study describes the assessment of quality attributes against Safety, Efficacy, PK/PD, and Immunogenicity. For each quality attribute, the Impact and Uncertainty are assessed and the product of these values is calculated as the Criticality with the maximum value assigned as the overall Criticality.
This new QbDVision functionality now allows Safety, Efficacy, PK/PD, and Immunogenicity to be defined as GAs and the Risk Ranking feature to be employed for the risk assessment of each quality attribute against these GAs. As a final step in the workflow, risk reports can be automatically generated to visualize the critical quality attributes of the product and how these attributes affect specific aspects of patient safety and efficacy as required in ICH Q8.
Availability
These features are available today in the Standard and Sandbox environment. Customers of our validated product will see them in the Electronica release, which is expected in January of 2021.