The final chapter at last!
On behalf of my QbDVision team, I hope you’ve enjoyed the journey we’ve taken through the rapidly evolving world of data governance for the drug development lifecycle.
We’ve covered a lot of ground! Together, we’ve taken a look at the scale of our industry’s data challenges, major barriers to data-centric drug development, promising frameworks for industry development data, and finally, some practical ways CMC programs can put those frameworks to use.
So what’s the last stop on this journey? Well this is a story about CMC, after all… so what else would we do but bundle it all together and loop in Regulatory?
In the final chapter of our story, let’s take a look at how the regulatory environment is shifting in response to today’s deluge of drug development data—and how regulators around the world are building awareness that, ready or not, structured submissions are on the way.
(Oh, and how one platform can help drug developers jump right into the “ready” line.)
Our evolving industry is ready for a new regulatory framework
If you missed Sue Plant’s fantastic presentation at the 2023 Digital CMC Summit, now’s the perfect time to stream it. She takes a bracingly no-nonsense look at how quickly regulators are evolving their pathways for the digital age—and how much work most drug sponsors and CDMOs must do to catch up with these expectations.
Was our industry caught by surprise? Hardly: the digital momentum Sue describes has been building for quite some time now. And today, it’s a real force that’s already reshaping how information moves through CMC programs industry-wide.
Reaching this point has been a journey for the CMC ecosystem: from electronic document storage in ERP and eQMS, to MES systems, to data platforms specially equipped for the challenges of spreadsheet-based data management and CPV. New electronic lab notebooks, LIMS, data lakes – all these technologies have proliferated in the industry.
And now, new solutions have come online that can serve as the information backbone drug developers need to enhance data integration, increase traceability and facilitate knowledge capture.
Structuring CMC datasets: “Defining what things are called and what they really mean – that really matters.”
If you missed Sue Plant’s fantastic presentation at the 2023 Digital CMC Summit, now’s the perfect time to stream it. She takes a bracingly no-nonsense look at how quickly regulators are evolving their pathways for the digital age—and how much work most drug sponsors and CDMOs must do to catch up with these expectations.
Was our industry caught by surprise? Hardly: the digital momentum Sue describes has been building for quite some time now. And today, it’s a real force that’s already reshaping how information moves through CMC programs industry-wide.
Reaching this point has been a journey for the CMC ecosystem: from electronic document storage in ERP and eQMS, to MES systems, to data platforms specially equipped for the challenges of spreadsheet-based data management and CPV. New electronic lab notebooks, LIMS, data lakes – all these technologies have proliferated in the industry.
And now, new solutions have come online that can serve as the information backbone drug developers need to enhance data integration, increase traceability and facilitate knowledge capture.
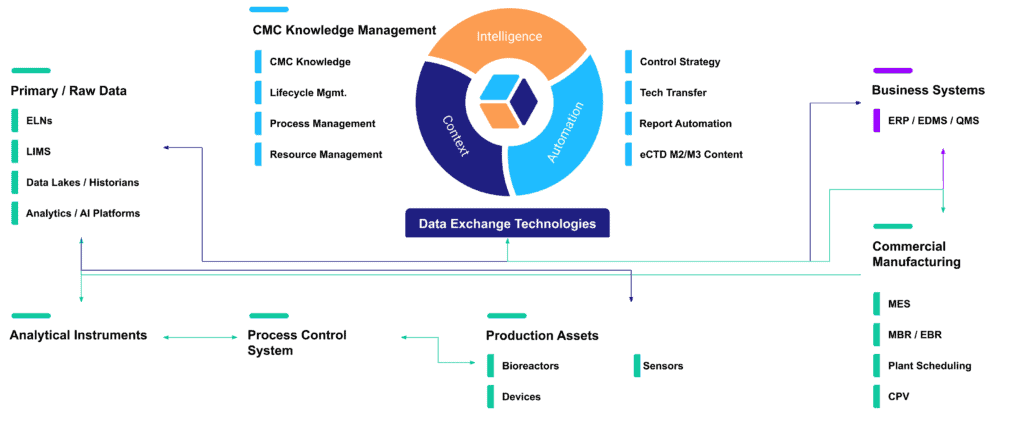
As these solutions span more and more of the CMC ecosystem—with increasingly efficient integration frameworks—the value-adding potential of drug development data has blossomed as well. Many industry stakeholders have been happy to allow this slow evolution to continue, one narrow capability at a time, gradually connecting workflows and data sources across their operations… but regulators…
Well, not as much.
Structured submissions are coming fast. And regulators are in the driver’s seat.
As Sue also noted at last year’s Digital CMC Summit, the age of unstructured, narrative regulatory submissions is closing fast. As numerous regulators have pointed out, legacy compliance and approval processes—with their heterogeneous structures, binders of “consolidated” information, and voluminous long-form PDFs—have become a major bottleneck that’s in dire need of a solution.
And what will that solution look like? To see what regulators have in mind, look no further than the FDA’s own KASA initiative: Silver Spring’s ongoing push toward Knowledge-Aided Assessments and Structured Applications.
The age of unstructured, narrative regulatory submissions is closing fast. Legacy compliance and approval processes—with their heterogeneous structures, binders of “consolidated” information, and voluminous long-form PDF—have become a major bottleneck that’s in dire need of a solution.
Explicitly billed as a “new approach that modernizes FDA’s quality assessment of regulatory drug applications,” KASA takes direct aim at outdated narrative submissions—and gives future applicants a firm push toward systematic, cloud-based submission processes based on highly structured data.
KASA has been underway for nearly a decade now. Internally, the FDA has already implemented this program for generics and is now extending it to other application types. In that time, the EMA was eager to jump on the trend: their fast-developing data quality framework has already reinforced KASA’s emphasis on “quality at the source,” and made it clear that drug developers seeking access to EU markets will need to show much greater maturity in their data governance.
Industry trade organizations have also been quick to note the importance and implications of this trend, with the ISPE recently devoting an entire issue of their publication to the topic of Knowledge Management. Our colleagues at BioPhorum have opened a dedicated workstream on structured data and lifecycle management, while the team at NIIMBL has introduced an entire framework dedicated to assessing technological maturity at biomanufacturing organizations.
Of course, all this parallel activity begs an important question: how will the industry standardize and systematize tomorrow’s digitally-enabled submissions? What should harmonized, cloud-based review processes look like?
Luckily, the ICH has already signaled the answer.
ICH is adding to the momentum with its upcoming revisions to M4Q
M4Q(R2) – the first update to this crucial guideline in 20 years – is poised to deliver a sea change in how drug developers and regulators exchange compliance-related data. My colleague Sana Ahmed recently took a deep dive into this topic, and looked closely at how these changes zero in on some key industry challenges – especially the proliferating, largely unmanaged complexity of the data appearing in regulatory submissions.
Starting with their 2021 concept paper on M4Q(R2), the ICH astutely focused on 6 critical goals for modernizing data exchanges in regulatory review and approval pathways. But it’s the fifth that has especially important implications for improving submission and assessment efficiency:
- “Enabling efficient use of digital tools for submission and assessment and preparing for the closely linked, upcoming ICH guideline on structured pharmaceutical quality submission”
As a panel of experts noted in their industry perspective on M4Q(R2), the real thrust of this goal is digitally overhauling CTD Module 2 (Quality Overall Summary) and Module 3 (Quality). All too frequently, these foundational components function as depots for unstructured, poorly navigable CMC data. M4Q(R2) is a firm push toward the maturity regulators are looking for: rigorously structured CMC data in consistent digital formats.
So the regulators are ready and the guideline revisions are in the works. What’s next for drug developers that want to prepare for tomorrow’s structured data, electronic compliance ecosystem?
Excellent question with a ready answer.
QbDVision is already a step ahead – and you could be too.
Adapting to any new regulatory paradigm can be a daunting proposition, especially when it involves fundamental shifts in how an organization manages core resources like its data. But fortunately for drug developers, they already have access to an off-the-shelf solution that can make that transition nearly seamless.
QbDVision, after all, is a Digital CMC platform purpose-built to centralize, structure, and steward drug development across the product life cycle, from IND to commercial manufacturing. Those core functions are ideal for any life science organization seeking to prepare for the coming transformation in today’s regulatory landscape.
Structured submissions? QbDVision structures data by design. Streamlining creation of templated regulatory modules? In QBDVision, that’s as simple as using Smart Content Authoring to format, generate, and export the relevant documents – all in just a few clicks. Integrate risk management best practices? Our platform’s data structure is literally built on industry-standard QbD taxonomies and ontologies – so compliance happens with every keystroke.
Organizations across the industry are already seeing the benefits:
- Power users at Xeris Pharmaceuticals used QbDVision to get CMC off the critical path for key development steps, shaving 6-12 months off their tech transfer timelines.
- After consolidating all their CMC data on QbDVision, a major generic manufacturer in the Middle East was able to reduce their compliance reporting time by 95%.
- Digital leaders at both strategics like Bayer and emerging CDMOs like VernalBio have turned QbDVision into the hub of their connected CMC ecosystem, and are already seeing the benefits in their streamlined workflows.
So structured regulatory submissions: That’s one reaper you don’t need to fear. QbDVision is built for the needs of tomorrow’s high-performance drug developers, and can put your CMC program on the path to regulatory efficiency from your very first login.
But enough of my sales pitch (you knew it was coming!). Let’s take one last look back at everything we’ve learned across all 5 chapters of this series – and what it says about the future of drug development data.
Data governance & integrity: What we’ve learned and where we’re headed
Today’s drug development industry is at a major inflection point: the shift from accumulating vast amounts of data to leveraging that resource in transformational ways. Here’s what that means for life sciences organizations today, tomorrow, and in the industry’s immediate future:
- The data deluge will continue: Without a doubt, drug development data will continue its exponential growth, flooding businesses with information, straining data governance, exposing cracks in data integrity, and driving an urgent need for strong, scalable data frameworks.
- Data culture needs to be a top priority: As many drug developers have already discovered, true data integrity isn’t a program you can implement. It’s the outcome of data-centric workflows, behaviors, and business culture. Fostering that environment will be an essential step for any life science company that’s serious about data governance.
- FAIR data principles are now indispensable: They frame a functional and contextual data paradigm that’s ideally suited to supporting data governance and integrity. All these principles need is well-defined, industry-relevant taxonomies and ontologies to build rich data and metadata schemas.
- Regulatory agencies will be big transformation drivers: They understand the need for structured data paradigms, and are already actively pushing regulated businesses toward cloud-based technologies that will enable better data governance and integrity.
- As always, the ICH will provide critical guidance: The upcoming changes to ICH M4Q have already delivered much-needed clarity on how the industry can move toward structured regulatory submissions. Coupled with QbD principles, the ICH’s evolving guidelines also offer all the taxonomies and ontologies needed to support a FAIR approach for CMC data and data quality.
So there you have it: it will be a challenging but exciting time as our industry adapts and evolves to a new, data-centric era. I for one am confident that we can navigate this new transition with all the scientific creativity, procedural precision, and organizational collaboration we aim to be known for. And I look forward to seeing where that will lead us.
And that, as they say, is a wrap. Stay tuned for more expert insights coming soon, from AI prep, to implementing product lifecycle management (PLM), and much, much more. The future is coming faster than ever, and the QbDVision team is ready to help shape it!
Catch up on the rest of our series on data governance and integrity:
GET IN TOUCH
Ready to prepare for tomorrow’s regulatory submissions?
Reach out to learn how we can help you build the data foundation you need to transform your compliance workflows.