Television series often start to get tired as the seasons progress, but not with the QbDVision Software Release Series. As this story progresses, it only gets more exciting and each episode will keep you on the edge of your seat!
The theme of Season 8 is Digital Leverage. The best way to describe this concept is by analogy. Think about a road trip you took 15 or 20 years ago. You probably pulled out a paper map or printed some directions from a website. You checked the traffic report on your TV or radio. And, you followed the signs as you drove to get to your destination. A disconnected and reactive approach to travel.
The world is very different today. Now you enter an address on your phone and it speaks to you with step-by-step directions to get there with layered in traffic data and building/landmarks in order to get there efficiently and find that fast-food meal you are craving at the end of a long day of driving.
How did this happen? Satellites digitized the earth with multiple layers of structured data and then added in more layers of information like cell phone tracking. These layers of structured data were then combined together to give you the integrated value that comes with digitized maps. This is Digital Leverage.
QbDVision is doing the exact same thing for CMC operations over the development lifecycle and this release of QbDVision, entitled Hip Hop, brings even more digital leverage to your CMC operations.
Here’s a snapshot of this season’s episode lineup
Episode 1: Digital Control Strategy
Episode 2: The Evolution of Acceptance Criteria
Episode 3: I Want To Be Dynamic
Episode 4: You Are Out Of Order!
Episode 5: The Document Connection
Episode 6: Don’t Forget The Small Stuff
Season Finale: Integration Management (What is that?)
Grab your popcorn (don’t forget the butter) and let’s get started!
Episode 1: Digital Control Strategy
The development of new therapies is a complex process that requires the convergence of multiple workstreams to tell a story about control. While regulators are very focused on the safety and efficacy of these therapies, they are equally focused on product quality. Product quality is a function of how the manufacturing process and production facilities are controlled to produce the drug substance and drug product per the specifications and regulatory requirements (see ICH Q8(R2) Section II.E).
QbDVision uses the digital leverage concept to simplify the development of a systematic control strategy. New features include:
- Tagging product and process variables how they will be controlled (e.g. Release Testing, Monitoring, Characterization, In-Process Testing, and more!).
- Tagging quality attributes to identify which are stability-indicating.
- Scoping final quality attributes to Drug Substance or Drug Product (DS/DP).
- New reports for Product Quality Attributes, Product Release Specifications, and Product Stability Testing Panel.
- Categorizing Control Methods as Analytical Methods, Monitoring, PAT, SOP/Training, and Facility Class.
- Linking Materials and Control Methods to multiple compendial standards.
- Merging Drug Substance and Drug Product categories for Materials and adding more specific categories for a better description.
- Automating Control Strategy Reporting:
- Product Quality Attributes
- Product Release Specifications
- Product Stability Testing
- In-Process Quality Attributes
- Characterization Testing Reports
- Process Parameter Control Strategies
- Material Attribute Control Strategies
Wow! How much time will this save you managing this information manually? That’s the beauty of digital leverage when applied to CMC. Our next release will build on this structure to enable broader visualization and reporting to enable a systematic approach to the definition, justification, and communication of the overall control strategy.
Episode 2: The Evolution of Acceptance Criteria
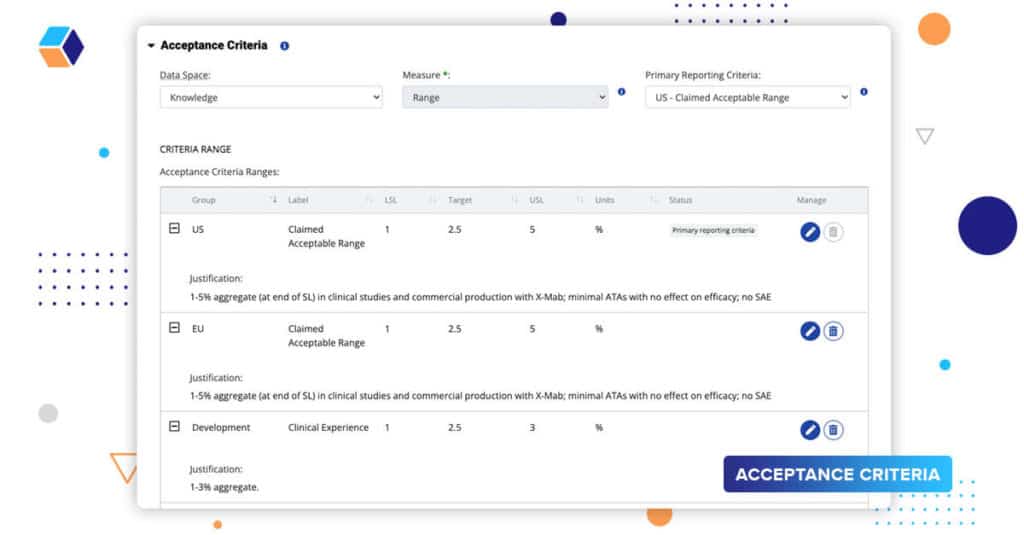
As the team moves through their development stages, they’re gaining a richer understanding of their product and process by assessing prior knowledge, doing in vitro studies, and executing process characterization. We have added new capabilities to dynamically manage acceptance criteria.
- Product Ranges – set up submission ranges, in vitro, animal studies, ranges from prior knowledge, and more for CQAs.
- Process Ranges – set up NOR (normal operating range), PAR (proven operating range – ICH Q8 R2), validation ranges, and more for CPPs and CMAs.
- Grouping Criteria – Acceptance criteria groups allow users to logically arrange similar ranges, for example, separate product acceptance criteria for US and EU submissions
Episode 3: I Want To Be Dynamic
Don’t we all! Who wants to be static? That’s no fun! Initially launched with our tech transfer functionality, we’ve expanded the representation of information in dynamic tabular views. We have extended the concept of the dynamic table to all the information in the Product and Process layers for any project. Now, you can create custom views and exports of all your product and process information. Whoa!
Functionality in the dynamic table view includes:
- Excel-like Power in your browser. Select columns, sort, and filter your information so you can customize your views and see what matters to you most.
- Shareable views. Once you have set up your table to focus on the information that matters, share the specific URL with coworkers to see the same view.
- Smart exports. Export what you see on the screen and take it with you, including your filters and sorting.
Episode 4: You Are Out Of Order!
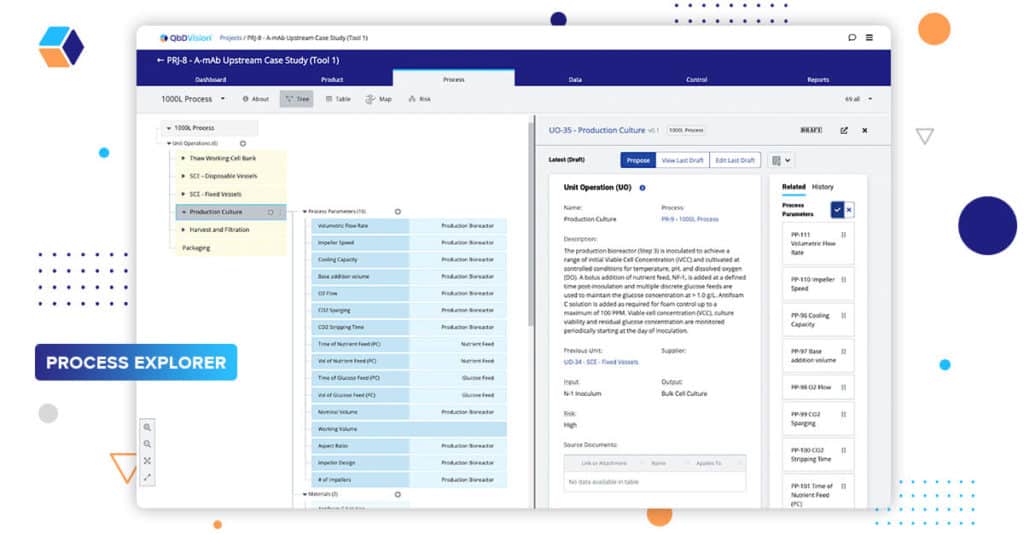
It’s time for the most anticipated episode of the series. Our Process Explorer module tackles its most popular request: Defining order for process parameters.
QbDVision is the first vertically integrated knowledge management system for pharma and biotech – bringing definition, risk, control, and analytics into one platform. Until now, when defining process parameters, the system didn’t specify the order of those parameters. Instead, it focused on grouping them with related components or materials in our knowledge graph. This grouping provided a contextual link that was important for thinking about your product and process. However, we recognized that process parameters have a natural order to them.
In our previous release, we introduced a new feature, Steps, to our Unit Operations. Steps provide a foundation for creating process order by providing a lower-level structure of the actions that take place in a process.
Now, we have added the ability to explicitly define the order of those process parameters inside their Unit Operations or Steps. Using a simple drag and drop interface, you can specify the order in which parameters occur within your process. This is, of course, in addition to linking them to the components and materials, as well as their downstream risks.
This capability brings us one step closer to defining the final recipe within QbDVision in alignment with the ISA88 process definitions which can subsequently be transferred to your manufacturing execution system (MES).
Episode 5: The Document Connection
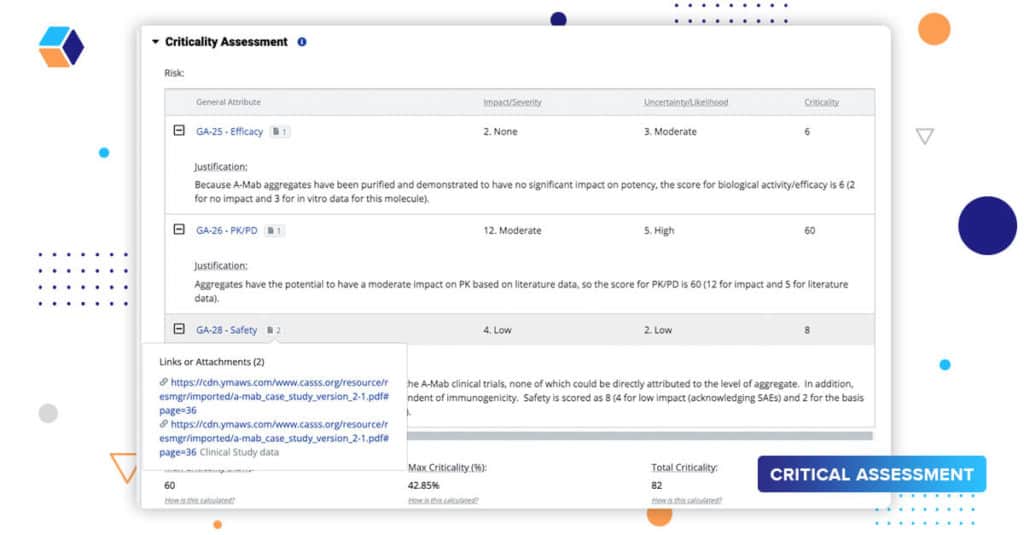
Whether you’re executing a product or process risk assessment, justifying your assessments is an important part of the workflow. Literature references, historical product knowledge, clinical information, DoEs, etc., are all part of a robust justification. Our source document widget allows users to attach or link to information – documents and reports stored in an eQMS, reports or analytics in a different system, or even other projects on our platform.
Linking this information not only provides a robust justification that is the basis for final submission, but also helps users understand and learn from prior knowledge. QbDVision serves as a single point of reference to multiple sources and workstreams. It helps bring together the big picture.
In this release, we’ve expanded this linking capability such that you can assign multiple source documents to their criticality assessments in final quality attributes using risk ranking and in our process parameters records.
Episode 6: Don’t Forget The Small Stuff
We all know the devil is in the details and Season 8 highlights the combination of many customer-driven improvements and new robust capabilities for product and process management. We are big believers in making sure our customers are happy and there is no better way to do that than to show them we are listening. You can always make a feature request through our customer support portal and we wanted to highlight some of the changes to the QbDVision platform that were requested by our loyal users.
Here are a few worth mentioning:
- Case insensitive imports – Uploading data into the platform just got a little easier with fewer restrictions on the upload process.
- Additional to supplier tracking, including additional comment fields and better tracking of virtual audits.
- New fields tracking the status of calibration development and tracking of a “Design Qualification (DQ)” state for Process Components.
- Additional project definitions for ‘Phase 2a’ and ‘Phase 2b’.
- Support for new Document Types in our QMS module including CAPA, Change Request, and Non-Conformance/Deviation
Season Finale: Improved Integration Management
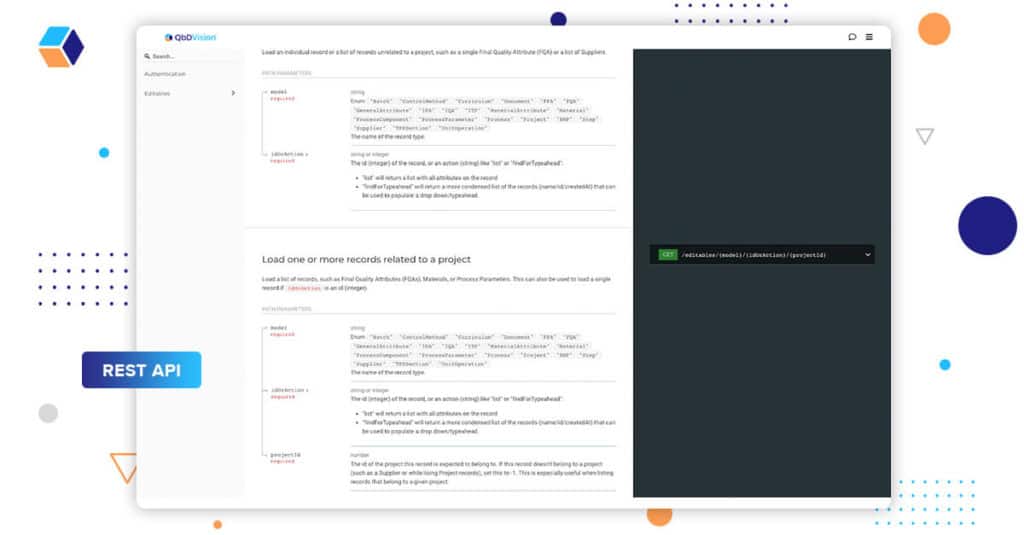
Season 8 ends with the appearance of the “Consultants” with some serious consultant “speak”. As the contextual layers of your product, process, and control strategy come together in QbDVision, it’s important that data and information are able to flow into and out of our system. Advanced users of our platform with technical capabilities can use our REST API infrastructure to programmatically access the information within QbDVision.
We’ve extended this capability to include:
- A user interface for generating API keys and managing their associated permissions
- Better online documentation for the API itself
- An Open API 3.0 compliant specification.
As we tease Season 9, we’re continuing to invest significantly in our product development in light of our recent funding announcement.
If you are new to QbDVision and are interested in trying out our solution, please reach out to learn more about exploring the platform’s expanded capabilities.