A Digital Approach to Continuous Process Validation
Authors: Lewis Shipp, Sana Ahmed, and Patrick Riordan
Have you ever wondered what validation looks like in the Validation 4.0 concept?
Let’s take a look at the different aspects and milestones of what this entails, by using a Quality by Design (QbD) approach with knowledge and risk management as enablers. To demonstrate these key concepts, let’s first take a look at a video that explores the ACE Case study in order to demonstrate how to create effective knowledge management in a digital system using a QbD approach following ICH Q8 through ICH Q12.
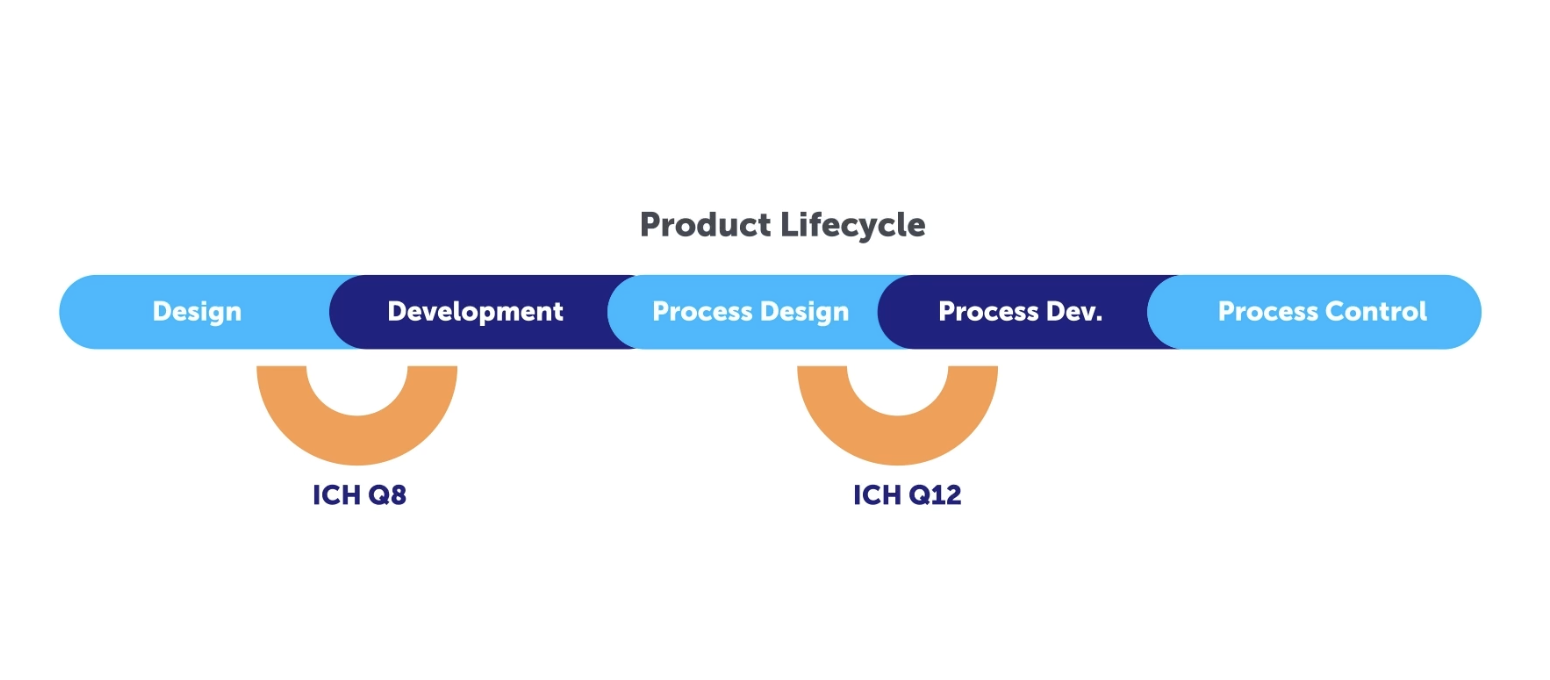
Validation 4.0 seeks to develop a cohesive harmonized, integrated, holistic, risk-based approach for process performance qualification that incorporates computer system validation building on the Pharma 4.0™ operating model. This includes a holistic control strategy that focuses on digital maturity and data integrity by design. This works hand in hand with Quality by Design which is meant to create a systematic approach to drug development, starting with pre-defined objectives, with emphasis on both product and process understanding. In addition to risk control based upon sound science and quality risk management.
The Current Landscape
Currently, the integrated approach for risk management through the patient, product and process is introduced both reactively and retrospectively. This is due to the overt focus on compliance, which should be automated, rather than quality. However, a holistic approach to knowledge management encompasses the identification, control, and verification of risks to end product quality and safety, in a continuous loop. This touches on what we may think of as them control strategy framework, which enables the prediction or simulation of impact in real time and the subsequent proposition of the necessary process adjustments . Moving away from reactive decision making, and into a new paradigm of developmental agility, built upon a holistic overview of production.
Quality by Design and its implementation are no longer optional, but mandatory, for achieving the aims of Pharma and Val 4.0 as well as, the transformation of our industry.
It is clear, from a regulatory and industry standpoint, that Quality by Design and its
implementation are no longer optional, but mandatory, for achieving the aims of Pharma and Val 4.0 as well as, the transformation of our industry. Digital tools that enable the continuous flow of data for iterative QbD and Knowledge Management as well as the ability to continuously demonstrate Control, and consequently facilitate a continuous state of Validation represent a great leap forward for Pharma.
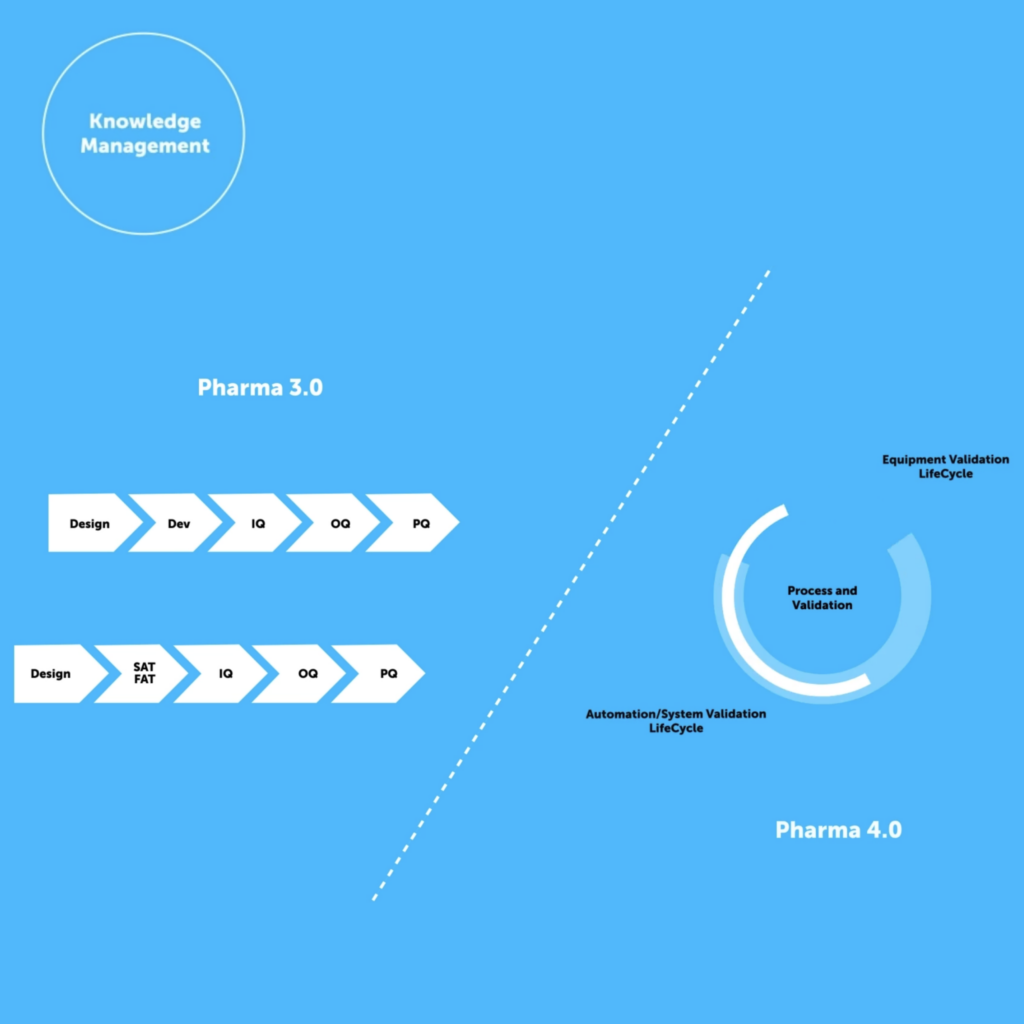

QbD and Val 4.0 Go Hand in Hand
QbD is an enabler of Val 4.0, as validation is based upon continued process control through iterative knowledge management and Quality Risk Management (QRM) from concept through to post-marketing. The achievement of Val 4.0 is predicated on approaching knowledge management both sequentially and methodologically, which is QbD, but it is often hindered by the lack of adoption and utilization of structured knowledge repositories.
The achievement of Val 4.0 is predicated on approaching knowledge management both sequentially and methodologically, which is QbD, but it is often hindered by the lack of adoption and utilization of structured knowledge repositories
Recently, an article was published from the Val 4.0 Working Group working group detailing the challenges of Oral Solid Dose (OSD) manufacture, and illustrating the relationship between Val 4.0 and QbD. The authors note that there are “Major issues in the traditional approach to validation are the lack of information on representative sampling; the difficulty of real-time monitoring and control; and the snapshot, rather than continuous manner, in which validation is performed.”
Swarbrick and Margetts also note that the key takeaway from their analysis of three OSD case studies is that “the principles of Validation 4.0 are proactive, not reactive. Under the old paradigm, traditional approaches were biased and based on selecting batches with the best raw material, operators, and analysts as a baseline to pass product for release. In Validation 4.0 and a truly QbD system, the use of data models, PAT, and feed-forward/feedback control establishes a process chronology and digital signature for comparison to past and future batches. Therefore, digitization and QbD allows for true validation of every batch.
The Case for Digital Tools
To illustrate the capability of a digital tool to demonstrate CPV, QbDVision works as a Digital CMC Platform and follows the Acetriptan CMC Tablet Case Study produced by the CMC-IM Working Group in 2008. The case study uses a QbD approach and embeds risk from the QTPP stage, through to scale up and optimization of the processes. The purpose here was to aggregate all this information into a central repository and import it to not only provide more context but to provide templates, tables, maps and charts/matrices that can be utilized throughout the validation lifecycle as outlined in the video below.
In the ever-evolving world of drug development, digital tools like QbDVision are creating a transformative path forward by combining digital innovation, effective knowledge management, and Quality by Design principles to shape the future of Continuous Process Validation.
As our industry embraces Validation 4.0’s holistic and risk-based approach, guided by the Pharma 4.0 model, the integration of QbD principles and digital technology adds depth and precision to the process. The insights gleaned from these collaborative efforts underscore the critical importance of knowledge management, proactive risk control, and real-time adaptation. By leveraging new technologies and new processes, our drug development era stands at the brink of a new era marked by agility, efficiency, and a commitment to product quality and patient safety.
GET IN TOUCH
Learn more about what QbDVision and Validation 4.0 can do for your drug development program.
Reach out to our team of experts any time to start a conversation about how you can protect your mission-critical product and process data.


























































