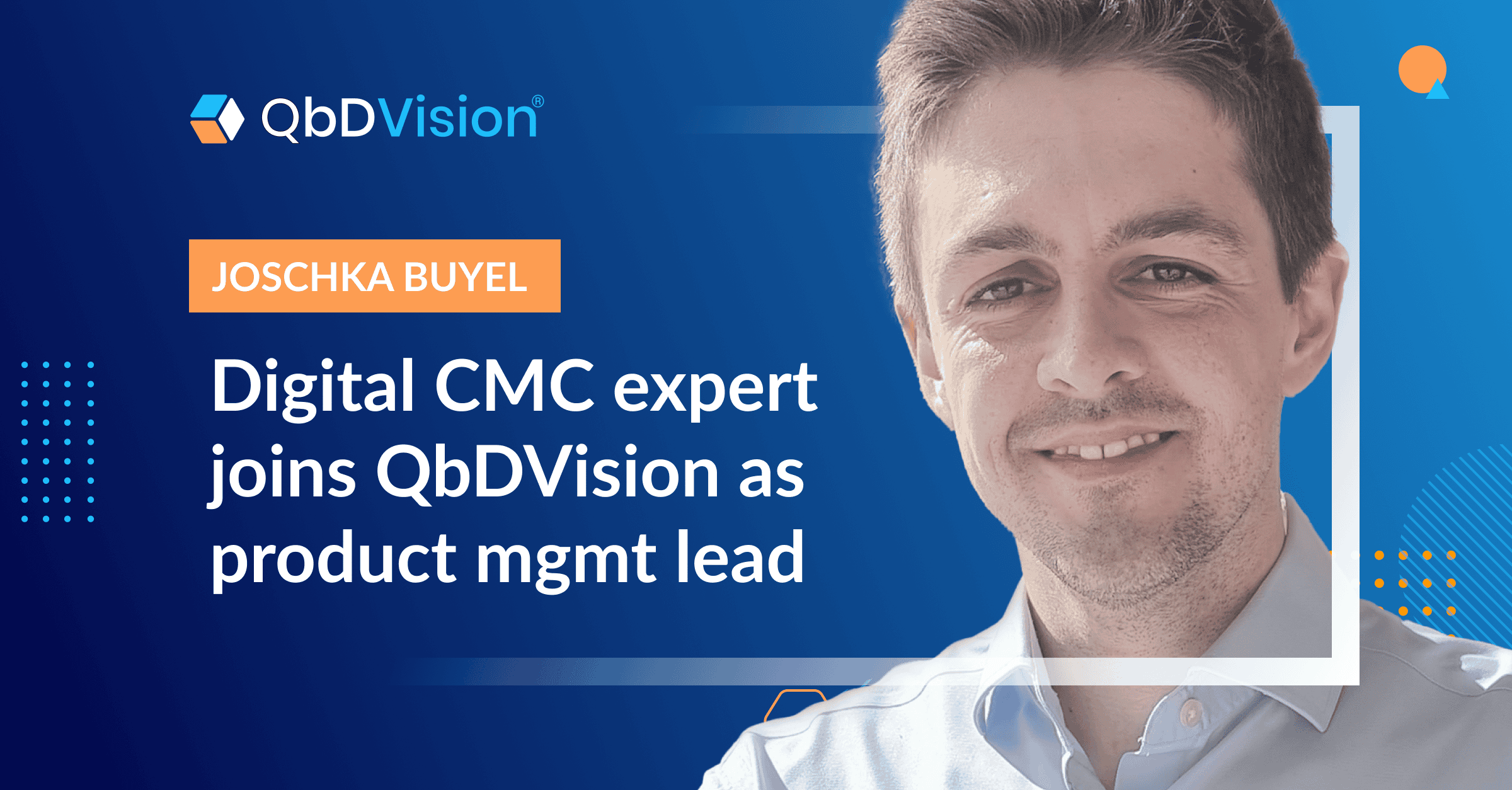
From multi-modal expert to product management leader, Joschka’s career is full of pioneering roles.
A biotechnologist by training, with a PhD in drug sciences, Joschka Buyel has spent his career at the digital forefront of the biopharma industry. And now, we’re proud to announce that he’s added a (we’re biased) exciting new entry in his uniquely impressive CV: Director of Product Management at QbDVision.
If you attended the 2022 or 2023 Digital CMC Summit, you know exactly why we’re thrilled to welcome Joschka to our team: He has a unicorn blend of deep domain knowledge, Digital CMC expertise, and hands-on deployment of our technology. In successful stints at both Bayer and Viralgen, he helmed implementation of QbDVision in some of the industry’s most advanced drug development programs.
That’s right: One of our top QbDVision superusers will be taking the reins of one of our most critical growth functions. And we couldn’t be more excited to see where he’ll guide it.
Here’s a sneak peek at where his focus will be!
“A one-stop shop”: What Joschka discovered as a QbDVision user
Joschka’s scientific and operational roots run deep. He started out as a process expert specializing in late-stage projects and preparations for drug filing, experience that helped him build world-class expertise in quality-by-design for drug development. And ever since, he’s had a keen eye on how the drug development and manufacturing process could run more efficiently.
His introduction to QbDVision came in 2020, when Bayer decided to adopt a central CMC data management tool as part of their digital roadmap. They ultimately landed on our platform, which Joschka notes was the only true solution in a space that has long been dominated by PDFs, SharePoint, Excel files, and other decentralized data management “systems.”
In his subsequent experience rolling out QbDVision — first at Bayer, then later at their satellite Viralgen — Joschka was quick to observe the software’s many advantages.
“It’s truly a one-stop shop,” he says. “That’s what I really love about the software. It’s one central knowledge management tool where all the development and manufacturing information is captured, and where transfers can be managed.”
That last point — tech transfers — is one that Joschka was particularly enthused about when he began working directly with QbDVision’s software. “It was the first time I was seeing the tech transfer gap assessment as a structured dataset,” he shares.
“It’s something that really excites me because there are a lot of benefits over an unstructured transfer. It’s not only time. It’s reusability, it’s the integrity of the information, it’s the ability to communicate exactly what you mean rather than creating the risk of misunderstanding—plus the ability to use the structured process definition in other tools like a Manufacturing Execution System (MES).”
"QbDVision is truly a one-stop shop. That’s what I really love about the software. It’s one central knowledge management tool where all the development and manufacturing information is captured, and where transfers can be managed.”
Structuring CMC datasets: “Defining what things are called and what they really mean – that really matters.”
Over the course of his career in drug development, Joschka has learned a thing or two about the importance of structure and standardization in CMC workflows. At Bayer, he was tasked with creating a taxonomy for the organization’s manufacturing processes.
“Defining what things are called and what they really mean,” Joschka observes, “That’s one of the key pieces to enabling structured datasets.” And as he’s quick to point out, structured datasets are essential for everything from future applications of AI technology, to automating regulatory reporting, to streamlining key handoffs and system integrations in the drug development cycle.
As Joschka also notes, data-related misunderstandings can happen at countless points in that cycle – and they’re more common than many think. They can start with variances as simple as using the word “container” in a tech transfer: Seems harmless enough, but someone on the receiving end may not know whether that container is closed or not. And in Joschka’s experience, if you’re not precise about what you actually mean, “it can quickly spiral into assumptions and misunderstandings.”
Crucial as it may be, structure is precisely what’s lacking in many of the existing CMC processes and workflows today. Take electronic laboratory notebook (ELN) systems, for example – which Joschka argues give users too much freedom to enter information. “If you can create a field for everything and name it however you want, you will not end up with structured data.”
For Joschka, that structure is one of the standout benefits of QbDVision’s software. “It streamlines the process of developing a new drug because all the tasks are basically predefined and you just follow them,” he observes.
And, Joschka adds, bringing structure to CMC data goes a long way toward avoiding costly miscommunications and assumptions downstream too. As he found throughout his experience rolling out QbDVision software, “You can be very explicit, especially when you use a taxonomy on top of it. And that’s a big de-risker.”
The results, as he saw first-hand, can be transformative for CMC programs currently drowning in unstructured datasets.
“Pharma is a slow ship”: Navigating the lag between technology advancement and adoption
Of course, even with the many merits of the software, adoption of new technology is often a bumpy road. While that’s true in any industry, Joschka observes, it’s especially common in drug development and commercial manufacturing, where barriers to change occur on multiple levels—from frontline scientists trained on notebooks to leadership with hundreds of priorities to balance. And he has his eye firmly on how QbDVision needs to navigate that challenge.
Some obstacles, of course, are simply factors of a regulated environment. “Once you file a drug,” Joschka notes, “any change that you make—how you manufacture it, how you capture data, etc.—will have huge implications on your existing filing. And that makes many stakeholders very change-averse.”
Adoption friction is especially common in drug development and commercial manufacturing, where barriers to change occur on multiple levels—from frontline scientists trained on notebooks to leadership with hundreds of priorities to balance. Joschka has his eye firmly on how QbDVision needs to navigate that challenge.
But as Joschka saw firsthand, reluctance to adopt new technologies can also come from users themselves: employees who have been doing their jobs one way for a long time and are reluctant to adapt, or management not supporting the change. Both groups, he notes, are important stakeholders for QbDVision – and ones he’s eager to engage as a fellow frontline user.
In the latter case, Joschka explains that it’s rarely because managers are against the new technology, but more often because they don’t see what’s in it for them if they adopt it. And for scientists, engineers, and other hands-on users, today’s inefficient, labor-intensive CMC processes often create a self-reinforcing cycle: those processes consume so much of potential users’ time that it limits their ability to learn and adopt new technologies.
In fact, dramatic underestimation of the amount of time lost to current workflows is one of the common misconceptions Joschka has found in his software rollouts.
“I often hear ‘Excel spreadsheets work just fine,’” he laughs. But more often than not, potential QbDVIsion users aren’t accounting for duplication of downstream work, time spent hunting and collating data, and many of the other inefficiencies endemic to today’s CMC programs.
And that’s a story he’s looking forward to telling many more biopharma companies.
The tides of change: Moving toward a more digitally integrated industry
Fortunately, while the barriers to change Joschka has encountered are significant, there are trends in the other direction too. “Software is becoming more and more important, and people are starting to understand that they need structured data,” Joschka says.
In part, that comes from the growing pressure from regulators to move toward structured submissions. But it’s also a result of the recent explosion of interest in AI models that require very strong datasets to train on.
Plus, Joschka has seen firsthand how current events can upend longstanding manual work processes and inefficiencies. “During the COVID-19 pandemic, people had to break through the very change-reluctant space in pharma to make things happen faster,” he notes. “It challenged a lot of existing workflows that are out there today. But speed and efficiency still won out.”
The bottom line: The change management process may be long, but there are still plenty of signs that point toward a more digitized CMC future. And when it comes to envisioning what that future could look like, Joschka is still long on the technology-enabled transformation that’s already gathering momentum — and on QbDVision’s trailblazing role in it.
“We’re here to make a lasting impact”: Joschka’s vision for what’s next
Looking ahead to his new role at QbDVision, Joschka is excited to help customers turn knowledge currently trapped in PDFs and spreadsheets into the invaluable enterprise resource it can be.
Like the rest of his team, he’s also looking forward to someday unlocking the value of AI in CMC workflows, and he’s well aware of the vital role QbDVision software will play in laying the foundation — in the form of structured data — to make it all happen.
Speaking of structure, you can expect Joschka’s expertise in taxonomy and standardization to remain at the heart of what he does in his new position. To that end, he’s already looking at ongoing developments, like the creation of a CMC ontology by Pistoia Alliance, and thinking about how any aligned industry standard can be integrated into QbDVision software in the future.
And while it might take a while to get there, Joschka has his eye on another loftier, industry-wide goal to work toward: the seamless integration of tools into a full data ecosystem. He notes that QbDVision is fully integrable today, but continuing to make that integration as easy as possible for customers remains a top priority.
We can’t wait to see how he’ll help guide us to those goals!
GET IN TOUCH
Ready to see what’s next on our product roadmap?
Connect with our team to learn more about where and how we’re expanding the impact of Digital CMC.